Since my last post in July the Marles-Wright Lab has grown with the addition of two new PhD students, Didi and Laura, who joined Kirsten and me in September. After a conversation with guest seminar speaker this term where both Laura and I struggled to sum up her project succinctly, I challenged everybody in the group to come up with a 100 word summary of their project that they could use at a Christmas party, or in casual conversation with an FRS.
Here they are (unedited) for your consideration.
Laura
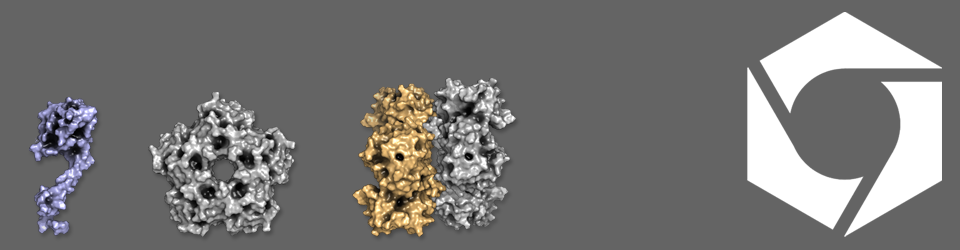
My project is based on bacterial microcompartments, which are proteinaceous structures that compartmentalize various metabolic processes such as fucose and rhamnose, or ethanolamine breakdown in a wide range of bacteria. The ultimate aim of my project is to synthesize an artificial fucose and rhamnose compartment in Clostridium phytofermentans, however to achieve this more information is needed about how the shell proteins and enzymes that make up these compartments are recruited and in what ratio are they combined. To try and answer some of these questions I will develop a shell protein localization sequence vector that will hopefully allow me to screen large numbers of signal sequences to find binding partners between microcompartment proteins.
Didi
Bacterial microcompartments (BMCs) are cytosolic, polyhedral organelles with a proteinaceous shell and enclosed enzymes, which protect the cell from volatile or toxic intermediates (propionaldehyde/acetaldehyde) or active oxygen. I focus on the microcompartments from Rhodospirillum rubrum. Through understanding how BMCs self-assembling we can modify them to contain potential therapeutic cargoes. To understand the protein assembly and metabolic pathway within the BMC, I will determine the substrate inducing the BMC production, followed by understanding protein components and interactions. Then I will propose a metabolic model of BMC based on transcriptional, protein and metabolic analysis. Finally, I will clone the BMC or construct functional empty microcompartments in vitro.
Kirsten
My research focuses on the most basic microcompartment, the empty shell. I am involved in engineering these minimal compartments consisting of shell proteins only to initially study their structure and the influence of tags on the formation and size of the compartments. Subsequently, we aim to design compartments filled with either molecular probes to further analyse their properties, or compartments containing different enzymes to study the effect of encapsulation on various enzymatic reactions. Apart from the engineering aspect of this project, I am involved in designing the relevant assays to study the compartments and their cargo using biochemical as well as microscopy based techniques.